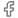IV Cannula Production Equipment for Efficient Medical Manufacturing Solutions
The Evolution and Importance of IV Cannula Making Machines
In the realm of modern medicine, the significance of intravenous (IV) cannulas cannot be overstated. These medical devices play a vital role in administering medications, fluids, and nutrition directly into a patient’s bloodstream. The demand for IV cannulas has surged, making the production process increasingly crucial in ensuring quality and efficiency. This is where IV cannula making machines come into play.
IV cannula making machines are advanced pieces of equipment designed for the automated production of cannulas. These machines incorporate cutting-edge technology and precision engineering to manufacture high-quality medical devices that adhere to stringent regulatory standards. The process begins with the selection of raw materials, typically medical-grade plastics that are biocompatible and safe for patient use.
The Evolution and Importance of IV Cannula Making Machines
One of the most significant advantages of using IV cannula making machines is their ability to produce large quantities of cannulas in a relatively short period. In a healthcare environment, where time is often of the essence, the rapid production capabilities of these machines can be a game-changer. Hospitals and clinics benefit from a steady supply of readily available IV cannulas, which ultimately facilitates better patient care.
iv cannula making machine

Moreover, the automation of the manufacturing process significantly reduces labor costs and human error. In the past, the manual assembly of cannulas was time-consuming and fraught with risk. Today, automated machines can not only increase production rates but also enhance the precision of each cannula, leading to improved safety profiles for patients. By minimizing the risk of defects or inconsistencies in the manufacturing process, healthcare providers can trust that the products they use will perform reliably.
However, with the increased reliance on technology comes the necessity for regular maintenance and calibration of IV cannula making machines. It is imperative that manufacturers adhere to maintenance schedules to ensure optimal performance and longevity of the equipment. Neglecting these aspects can lead to product recalls and safety concerns, which not only impacts manufacturers financially but can also have dire consequences for patient health.
The importance of adhering to international quality standards cannot be overlooked. IV cannula making machines must comply with regulations set forth by various health authorities, such as the FDA in the United States and the EMA in Europe. This regulatory scrutiny ensures that manufactured cannulas meet safety and efficacy standards before they are distributed for use in healthcare settings.
Looking to the future, the evolution of IV cannula making machines is likely to continue at a rapid pace. As technology advances, we can expect the emergence of even more sophisticated machines equipped with features like artificial intelligence and machine learning. These innovations may enable manufacturers to predict maintenance needs, improve production efficiency further, and even customize cannulas based on specific patient or procedural requirements.
In conclusion, IV cannula making machines represent a pivotal element in the healthcare supply chain. By leveraging advanced technology to streamline the manufacturing process, these machines help facilitate the production of high-quality medical devices that are essential for effective patient treatment. As the healthcare landscape continues to evolve, so too will the machinery that supports it, paving the way for enhanced medical care and improved patient outcomes.
-
High Frequency Straight Seam Welded Pipe Production Line-BzZhou Xinghua Machinery Equipment Manufacturing Co., LTD.|line pipe steel&welded gas pipeNewsJul.30,2025
-
High Frequency Straight Seam Welded Pipe Production Line-BzZhou Xinghua Machinery Equipment Manufacturing Co., LTD.|High Precision&Automated SolutionsNewsJul.30,2025
-
High Frequency Straight Seam Welded Pipe Production Line - BzZhou Xinghua Machinery Equipment Manufacturing Co., Ltd.NewsJul.30,2025
-
High Frequency Straight Seam Welded Pipe Production Line-BzZhou Xinghua Machinery Equipment Manufacturing Co., LTD.|Precision Welding, High EfficiencyNewsJul.30,2025
-
High Frequency Straight Seam Welded Pipe Production Line|BzZhou Xinghua|Precision Welding&EfficiencyNewsJul.30,2025
-
High Frequency Straight Seam Welded Pipe Production Line - BzZhou Xinghua|Precision Engineering&EfficiencyNewsJul.30,2025